Understanding GLP-1s and their dynamic marketplace and workplace trends
 With the availability of newer treatment options for weight management – glucagon-like peptide 1 (GLP-1) medications – metabolic health is in the news, shining a light for the public on the impact of weight on overall health.
With the availability of newer treatment options for weight management – glucagon-like peptide 1 (GLP-1) medications – metabolic health is in the news, shining a light for the public on the impact of weight on overall health.
When evaluating patient well-being, physicians are increasingly reviewing metabolic health as an indicator of future health risk. Metabolic health factors – including a patient’s cholesterol profile, waist circumference, blood pressure and blood glucose – are interconnected. Obesity is a multi-faceted medical condition that typically manifests as a result of several factors, including genetic makeup, use of certain medications, lack of physical activity, excessive calorie consumption and sleep patterns.1 There are over 200 conditions associated with obesity, including cardiovascular disease, diabetes, fatty liver disease and cancer.2 It is well documented that even a 10% reduction in body weight has health benefits.

Emerging Marketplace Trends
The GLP-1 market continues to be dynamic with five key emerging trends that employers and other stakeholders should consider, as they make actions that align with their coverage philosophy and population needs.
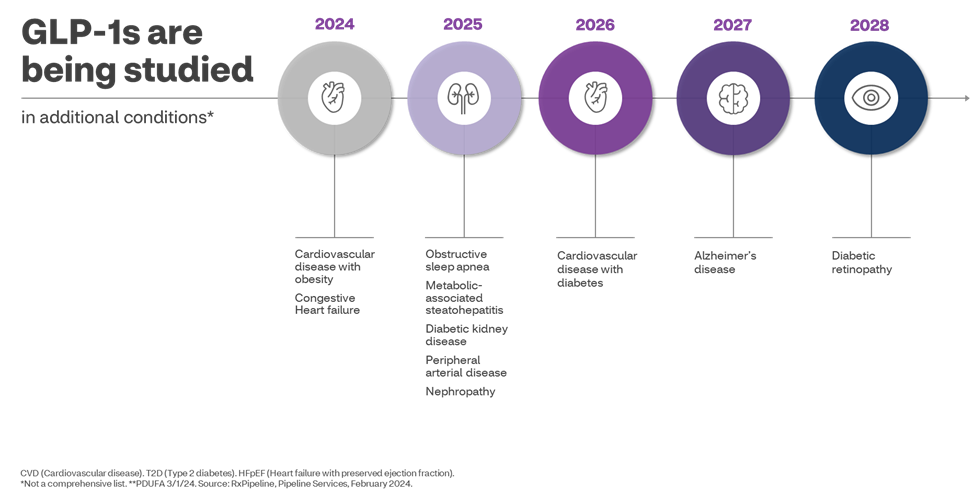
- Beyond Diabetes and Obesity: Researchers are studying numerous additional indications that could expand the use of these medications beyond diabetes and obesity. In March, Wegovy was approved by the Food and Drug Administration (FDA) to reduce the risk of major cardiovascular events such as death, heart attack, or stroke in adults with known heart disease. Early results from ongoing clinical studies are indicating the GLP-1 medications may successfully treat numerous other conditions including non-alcoholic fatty liver disease (known as metabolic dysfunction-associated steatohepatitis or MASH), sleep apnea, heart failure, diabetic kidney disease and Alzheimer’s disease.
- Growing pipeline of drugs: There is a robust pipeline of new medications being studied for the treatment of diabetes and obesity. Clinical trials are evaluating these new products alone and in combinations not currently available.
- Access and affordability: The high cost of GLP-1 drugs remains a significant hurdle for patients and payers. The manufacturers of these drugs have priced them at more than $1,000 per month, and limited competition in the space is putting them out of reach for many patients and creating financial burden on healthcare systems.
- Increased competition: Even with new medications expected to launch in the coming years, based on the prices of currently marketed products, it is unlikely that brand product pricing will decrease significantly in the next several years. We don’t expect significant cost relief from generics in the near term. Victoza – a GLP-1 for diabetes – is expected to lose patent protection in 2024. Saxenda – the same medication as Victoza but approved for weight loss – is expected to have patent protection until ~2027.
- Long-term efficacy, safety & duration: While GLP-1s have shown impressive short-term results, the long-term picture remains blurry. Limited data exists about the impact beyond weight loss when GLP-1s are used in patients without a diagnosis of diabetes. Extended clinical trials and real-world data are crucial to understand their lasting impact on weight, metabolic health, and potential side effects.
3 Key Considerations for Plan Sponsors
As you evaluate your plan benefit philosophy, including whether or not to cover the use of these drugs for weight loss, and prioritize what is most important to you, consider three key areas: Coverage, Cost and Care.
Coverage: consider your population and your benefit portfolio objectives.
Plans covering these agents consider coverage as:
- an investment into the health of their population;
- potential for lower medical spend in future years;
- an employee retention/ recruitment tool;
- the potential for better health to positively improve productivity, absenteeism and presenteeism.
Those excluding medications for weight loss may be doing so due to budget constraints.
Cost:
When plans cover these medications, rigorous utilization management is strongly recommended. Most plan sponsors cover these medications only for FDA labeled/clinical compendia-supported conditions. Formulary strategies also effectively manage client costs by reducing the net unit cost for payers.
Care:
Members taking these medications are more successful in losing weight when supported with diet and exercise. For plans that cover medications for weight loss, care management strategies, including member education, help optimize investment in weight loss medications and support patients in their journey towards better health.
At CVS Caremark, we recognize that management approaches to GLP-1s are not one-size-fits-all. The evolving metabolic health landscape and increasing employee expectations require a holistic approach to help contain costs while helping to preserve clinical quality and organizational goals.
Daniel Knecht, MD, MBA
Vice President & Chief Clinical Innovation Officer
CVS Caremark
LinkedIn: https://www.linkedin.com/in/dan-knecht-md-mba-4739b313/
1- https://www.cdc.gov/obesity/php/about/risk-factors.html?CDC_AAref_Val=https://www.cdc.gov/obesity/basics/causes.html
2- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7884814/
3- https://www.ajmc.com/view/fda-approves-diabetes-drug-tirzepatide-for-chronic-weight-management

